AsiaTechDaily – Asia's Leading Tech and Startup Media Platform

JiHee Jung, CEO of MEDIAIPLUS, Spearheads Transformation of Clinical Trial Data; Aims to be the ‘Amazon of Healthcare’
Seoul, known for its vibrant atmosphere and active clinical trial landscape, has long been a hub for cutting-edge medical research. However, despite the immense volume of valuable data generated from these trials, searching and collecting relevant information has proven to be an uphill battle due to limited digitization efforts.
Enter MEDIAIPLUS, a company determined to revolutionize the clinical trial data landscape. Under the visionary leadership of JiHee Jung , MEDIAIPLUS aims to bridge the gap between data availability and accessibility. With their innovative solutions, they aspire to transform how researchers, medical professionals, and stakeholders interact with clinical trial data, unlocking its full potential for advancing medical knowledge and patient care.
JiHee Jung recent presence at the VivaTech European Startup Festival has only solidified her position as a trailblazer in the industry. JiHee Jung shares her journey, vision, and the impact MEDIAIPLUS seeks to make in the clinical trial data field.
Q. Can you tell us why you established MEDIAIPLUS?
Our focus at MEDIAIPLUS is on creating a service that offers customized clinical trial data for venture companies in preparation.
Having worked as a pharmacist in Korea and actively engaging in clinical trial-related responsibilities at an international pharmaceutical company, I recognized the obstacles domestic pharmaceutical firms and bio ventures encountered when preparing for clinical trials. This realization drove my decision to establish MEDIAIPLUS, aiming to tackle these challenges collaboratively and foster advancement in the industry.
Q. How has MEDIAIPLUS changed the scope of clinical trials and related services in Korea?
Since the inception of our business, we have observed many bio ventures engaging in the preparation phase for clinical trials. The most remarkable aspect is that we have gained customers through positive word-of-mouth despite not actively engaging in marketing efforts. This outcome holds great significance and meaning to us.
Q. What led you to choose Pangyo Techno Valley as the location to start a business?

Despite having the opportunity to select a different location, I found the prospect of starting a business quite overwhelming. The absence of fellow entrepreneurs around me and the multitude of uncertainties regarding how to embark on the startup journey added to my concerns. Before establishing MEDIAIPLUS in 2018, I had gained experience in a foreign company, familiarizing myself with its organizational culture. However, starting a business was a stark contrast to the structured environment I was accustomed to. The infrastructure needed to be improved, and I had to navigate the challenges of operating on a small scale while maintaining a rapid pace of progress.
During that time, entrepreneurship-related education programs were offered by the Gyeonggi Business & Science Accelerator (GBSA), operated in collaboration with Pangyo Startup Campus. Through these programs, I had the opportunity to learn essential information about starting a business. It wouldn’t be an exaggeration to say that by participating in these programs and naturally choosing Pangyo. As a result, I received tremendous support as an aspiring entrepreneur. From the early stages of being a prospective startup founder to the current growth stage, MEDIAIPLUS owes much of its success to the assistance received. It was indeed an invaluable resource for aspiring entrepreneurs, and I express my gratitude to the Gyeonggi Business & Science Accelerator (GBSA).
Q. What kind of assistance are you receiving from the Information Security Cluster, the building where you are currently located?


When it comes to clinical trials, security plays a vital role, and the management of data requires a well-structured system. The Information Protection Cluster is a supportive hub for startups in the security domain. Not only does it offer various opportunities for education and recruitment in information protection, but it also provides dedicated spaces for startups.
Q. MEDIAIPLUS was established with the help of various supports, and we would like to know more about what services your company provides.
MEDIAIPLUS currently offers two services. The first service is Medic, a data platform related to clinical trials.
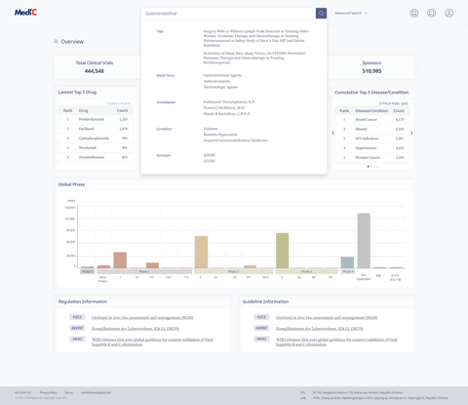
During the planning phase of clinical trials, a substantial volume of data is essential. Historically, gathering this data has been a labor-intensive endeavor involving the collection of information on various aspects, such as past clinical trials for specific diseases, ongoing trials by competitors, existing market products, and their pricing. Each piece of data had to be acquired and standardized for effective analysis, demanding significant manpower and time. However, our MediC service (MEDIAIPLUS Medic) offers a solution akin to digital craftsmanship or digital home industry. It consolidates a vast amount of data in a single platform, eliminating the need for arduous manual work and enabling users to conveniently access and analyze the information.
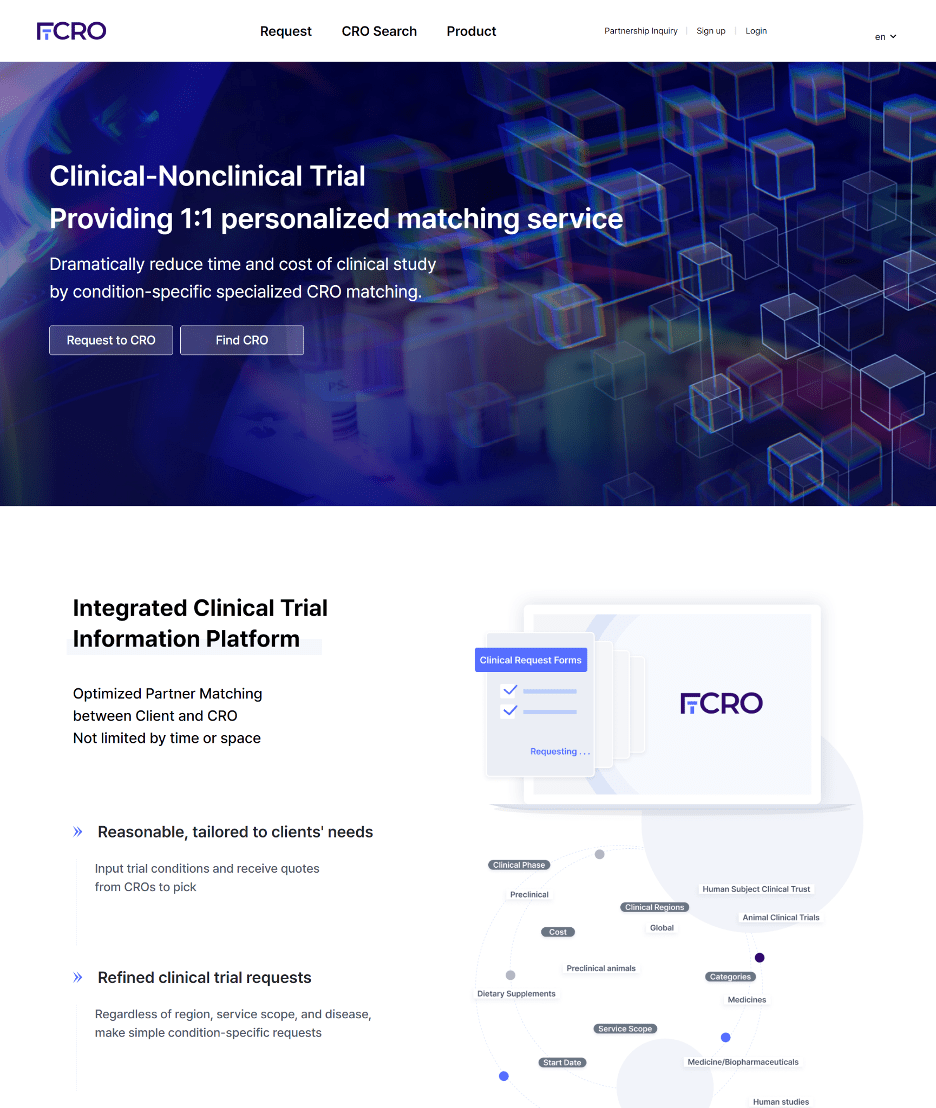
The second service is when conducting clinical trials, in cases where there is no infrastructure or when I need to conduct clinical trials quickly. Even if I have the infrastructure, I end up requesting the services of a contract research organization (CRO) specializing in clinical trials. However, searching for such a contract organization is still done analogously. It requires searching on the internet, asking around in the vicinity, or seeking assistance from consultants. What we do is digitize all of these processes into a database, allowing you to find them all at once and reducing the time required for the preparation process.
In other words, if we compare it to other startup industries, it is similar to the real estate services of “Zigbang” or “Dabang” in the * PropTech sector. But our company even provides an in-house service called FiCRO, which helps customers to find CRO.
These two services are currently being provided to bio-ventures, medical device companies, and health supplements companies. Additionally, we possess information on global clinical trials, making it available in South Korea and other countries. In fact, the US MIH currently has around 450,000 published clinical trial records, while we have access to 580,000 clinical trial records.
Q. What is the demand for MediaiPlus services in overseas markets?
Seoul is known as the city with the highest number of clinical trials in the world. When it comes to conducting clinical trials overseas, many people want to include Seoul. There are three main reasons for this. Firstly, Seoul is home to the Big Five hospitals. Secondly, South Korea has no time difference, making it convenient for patients from other regions to travel and participate. Lastly, the fast-paced culture in South Korea greatly aids in conducting time-sensitive clinical trials. Therefore, there is potential for the utilization of domestic clinical trial data even in overseas settings.
Q. You have been participating in various events to expand into overseas markets and recently attended Viva Technology. How was the response from overseas and European customers?

VivaTech is an annual technology conference dedicated to innovation and startups, held in Paris, France, from June 14 to 17 of this year. The event had a highly energetic atmosphere, with a warm and inclusive culture that was evident. The participants worldwide were receptive and encouraging, showing a willingness to collaborate. It felt authentic to share data with investors and companies, and I had the opportunity to form valuable connections.

Thanks to that, we established an MOU with StartingBloch, a French startup specializing in patent information, at this event. Through this MOU, we expect to reduce the time and cost of searching for patent information in clinical trial-related data and data required for new drug development. Additionally, through European networking, they will promote our company, and in turn, we will promote StartingBloch in Korea and Asia, which can lead to business expansion. Participating in VivaTech has been a great networking experience, and we highly recommend it to domestic startups as well.
Q. If we were to choose three major achievements from VivaTech, what would they be?

Visit of the Minister of the Economy of Luxembourg and officials to the MEDIAIPLUS booth at VivaTech (Image provided by MEDIAIPLUS) We confirmed the potential of our service in the international market, which was the most significant outcome for us. The key significance of participating in international exhibitions is the opportunity to establish ongoing connections with various buyers. At this event, we observed a great deal of interest from French CRO companies and bioventures in data curation related to clinical trials.
In particular, the representative from Amgen, a global pharmaceutical company, visited our booth and showed interest, which allowed us to feel that MEDIAIPlus products were well-received in the market. It was an opportunity for market validation.
Secondly, as a startup, we have limited manpower to handle all tasks. Therefore, it is crucial for us to receive assistance from partner companies. Through the event, we met and established MOUs with collaborating companies, which is another achievement.
Lastly, we had the opportunity to converse with investors and high-profile investment firms we wouldn’t easily meet otherwise. In Korea, it is not easy to meet investors in prominent positions, but thanks to the event, we were able to meet them in person, present our products, and even schedule follow-up meetings, which is the third achievement.
Q. Your company successfully advanced into an overseas market in a short amount of time. When it comes to the entry into the foreign market, what support did your company receive from Pangyo Techno Valley?
Gyeonggido’s local government provides extensive global support for startups. For instance, they offer support programs for participating in overseas exhibitions and competitions, providing opportunities to showcase products and services on an international stage.
Moreover, through the Accelerating Program, Gyeonggi-do local government provides valuable assistance through mentoring and collaboration. We received significant support through this program, which helped us achieve positive outcomes. In fact, we were the only startup in its infant stage from Korea to advance to the finals of the competition. This achievement is a testament to the great support we received. We are sincerely grateful to Gyeonggi Province for providing us with such opportunities. Their contribution has been instrumental in our success.
Q. Where do you see your company in 10 years?
Our goal is to expand globally. We aim to attract a significant number of domestic clinical trials and establish ourselves as a cross-border entity that facilitates the overseas expansion of domestic biotech companies.
Our ultimate goal is to become a marketplace in the field of clinical trial data, similar to Amazon. The development of new drugs, medical devices, and health supplements operates within a virtually borderless market known as the “One Market.” While there are regulatory differences across countries, the essence of providing treatment opportunities to patients remains unchanged. We aspire to establish ourselves as a bio-industry player, functioning as an “Amazon” of the clinical trial data realm, enabling rapid access to treatment opportunities for a large number of patients and fostering the growth of the biotech industry.
Q. As a senior entrepreneur in the Pangyo Techno Valley, what is the advice you want to give to aspiring entrepreneurs?
Don’t start a business. I’m telling you this first because it wasn’t as easy as I thought it would be. Even though I had a job, starting a business was still very difficult.
There is a lack of infrastructure and a lot to learn in this field. If you are not prepared for these challenges, I would not recommend it. However, if you have a promising product and good people around you, I recommend participating in various programs to build your network. I also suggest considering becoming a co-founder as a starting point.